Background:
Von Willebrand Disease (VWD) is the most common inherited bleeding disorder, thought to occur in ~0.1% of the population. VWD results from a quantitative (Type 1 or 3) or qualitative (Type 2) defect in von Willebrand Factor (VWF), a multifunctional plasma protein involved in primary and secondary hemostasis. Diagnosis of VWD can be difficult due to pre-analytical variables, a wide coefficient of variation in testing, and incomplete penetrance. Treatment of VWD is aimed at replacement of the defective or missing protein through plasma derived or recombinant VWF, release of endogenous VWF through desmopressin (DDAVP) or clot stabilization with anti-fibrinolytic therapy. Though individuals with mild VWD and bleeding symptoms are common, less is known regarding individuals with VWD and a clinically severe bleeding phenotype.
Aims:
To characterize the bleeding phenotype and treatment regimens in patients with clinically severe VWD in the United States.
Study Design and Methods:
ATHN 9 is sponsored by the American Thrombosis and Hemostasis Network (ATHN) and is being conducted at ATHN-affiliated sites across the US. Participants were identified by the site investigators with the projected goal to enroll 130 individuals. Inclusion criteria were patients with severe VWD defined as type 3 VWD, or VWF:RCo, VWF:GPIbM or VWF:Ag≤ 30% or patients with "clinically severe VWD" defined by VWF:RCo, VWF:GPIbM or VWF:Ag ≤ 40% a with severe bleeding phenotype (need for recurrent use of factor concentrates) and prior enrollment in the ATHN dataset national surveillance data collection project. Patients with platelet-type or acquired VWD were excluded. Laboratory assessment including a standardized diagnostic battery, VWF genetic analysis, and inhibitor testing, was performed by a central laboratory. Bleeding was assessed using the International Society for Thrombosis and Haemostasis (ISTH) Bleeding Assessment Tool (BAT) (normal adult 0-4, normal score <18 years 0-2) and the Pictorial Bleeding Assessment Chart (PBAC) if applicable.
Results:
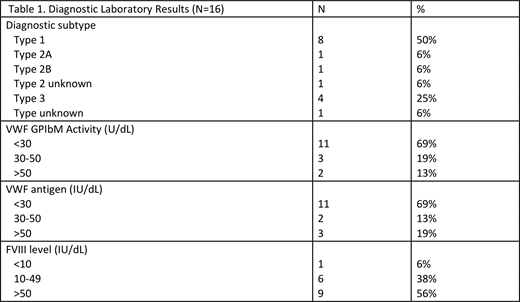
Initial data on 35 participants was analyzed. Most were adult (69%), female (66%), Caucasian (86%) and non-Hispanic (89%). Just less than half (16/35, 45.7%) have completed diagnostic testing (Table 1). Half of the patients had Type 1, a quarter Type 3, and the remaining had Type 2 or unknown. The majority of patients (69%) had VWF GPIbM activity <30IU/dL, while 44% had an abnormally low FVIII level as well. The majority (26/35, 74.3%) had a known family history of VWD. Slightly over half (19/35, 54.2%) had previous surgery. Few participants (4/35, 11.4%) reported the presence of a target joint at enrollment, ankle being most common. The bleeding phenotype was significant but variable with a mean ISTH BAT score of 10.6 (range 0-39). With the exception of the youngest cohort (0-5 years of age, mean BAT score of 6, range 3-8), bleeding scores increased with age and all participants had abnormal scores. The most commonly endorsed symptoms were epistaxis, heavy menstrual bleeding (HMB), and post-surgical bleeding. The PBAC was performed on 4/10 participants in reference to their last period with a median score of 36 and range of 0-112 (>150 is abnormal). The majority (3/4) of participants filling out the PBAC received VWF concentrate prophylaxis for HMB. The majority (23 participants, 66%) utilized factor concentrates for prophylaxis or on-demand treatment; six patients (17%) were on continuous prophylaxis, while 12 (34%) were on event-based or HMB prophylaxis while the remainder received episodic treatment. Participants most commonly used plasma derived VWF concentrate (93.9%) with the remainder using recombinant VWF.
Discussion:
Initial evaluation of 35 participants with clinically severe VWD demonstrated a predominance of mucosal bleeding with a minority of participants endorsing joint bleeds at enrollment. Despite abnormal ISTH BAT scores in all participants, PBAC scores were within normal range, likely reflecting appropriate management of HMB with most participants receiving VWF concentrate for HMB prophylaxis. In contrast to patients with mild disease where antifibrinolytics and desmopressin are frequently used, factor replacement was the most common treatment modality. Future analysis will focus on laboratory evaluation, bleeding phenotype, response to factor replacement therapy and quality of life.
Weyand:Shire: Membership on an entity's Board of Directors or advisory committees; Kedrion: Membership on an entity's Board of Directors or advisory committees; Aptevo: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees. Friedman:Alexion: Speakers Bureau; Bayer: Consultancy; Instrumentation Laboratories: Consultancy; Alexion: Consultancy. Haley:ATHN: Research Funding. Roberts:Sanofi: Consultancy, Speakers Bureau; Novo Nordisk: Consultancy, Speakers Bureau; Pfizer: Consultancy; Takeda: Consultancy, Research Funding, Speakers Bureau; uniQure: Consultancy; Octapharma: Consultancy, Speakers Bureau. Sidonio:Genentech: Membership on an entity's Board of Directors or advisory committees; Sanofi/Bioverativ: Membership on an entity's Board of Directors or advisory committees; Octapharma: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Uniqure: Membership on an entity's Board of Directors or advisory committees; Biomarin: Membership on an entity's Board of Directors or advisory committees; Catalyst Sciences: Membership on an entity's Board of Directors or advisory committees; Emergent Solutions: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Kedrion: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Octapharma: Research Funding; Grifols: Research Funding; Grifols: Membership on an entity's Board of Directors or advisory committees.
VWF concentrates used for heavy menstrual bleeding
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal